:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
Write definitions of:
Atomic radius
Electron Affinity
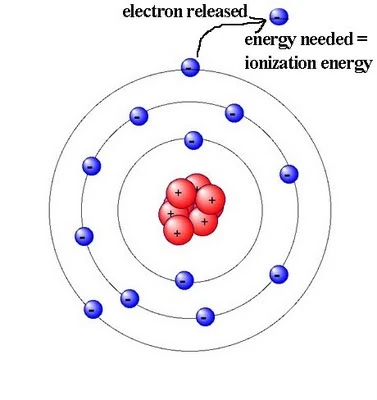
Ionization Energy
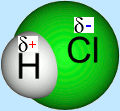
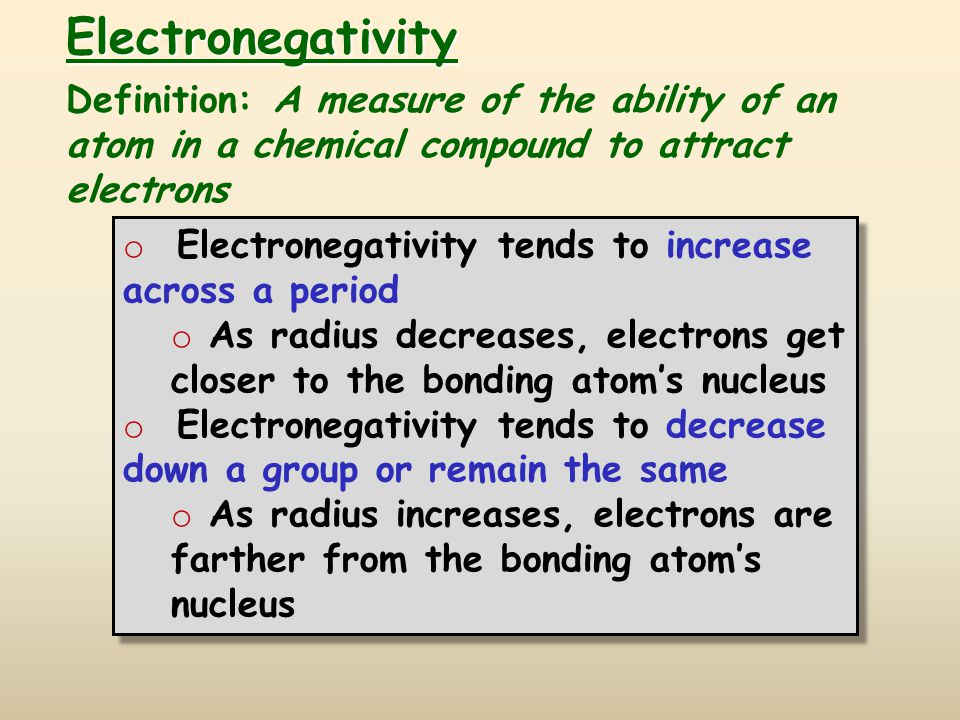
Electronegativity
More electron affinity results in more ionization energy. So, it is more difficult to lose an electron or separate an electron that is already in the atom.
Electronegativity increases across the period. The trend is opposite to that of the atomic radius.




No comments:
Post a Comment